Life on Earth and Mars: Was there life on the red planet?
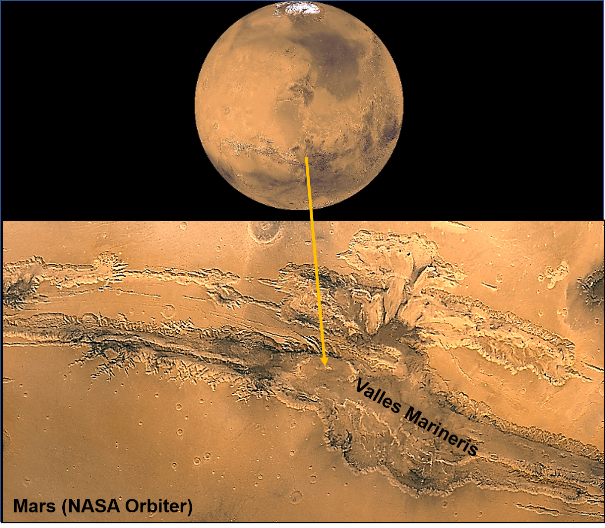
Evidence preserved on Mars indicates the planet was geologically active in the past. Examples are the extensive remains of volcanism (see Part 3, figure 2) and possible large-scale tectonic activity indicated by Valles Marineris (Figure 1). These features formed from heat generated by nuclear fission below the planet’s surface by radioactive decay.
Oxidation on Mars
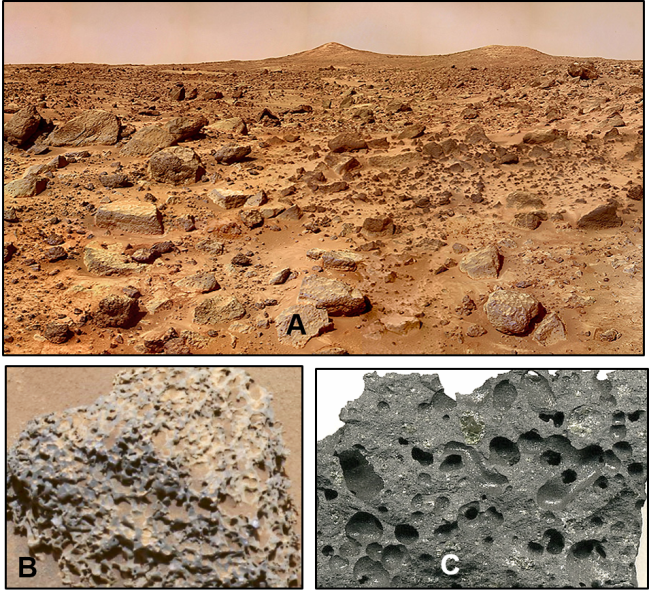
NASA has determined that much of the Martian surface consists of sedimentary and volcanic rocks along with surface sediments in which iron has been oxidized, or rusted. In areas that have been studied, the depth below the surface to which oxidation has occurred is only a few meters. It is not yet known when during Martian history this process occurred (Figures 2 and 3).

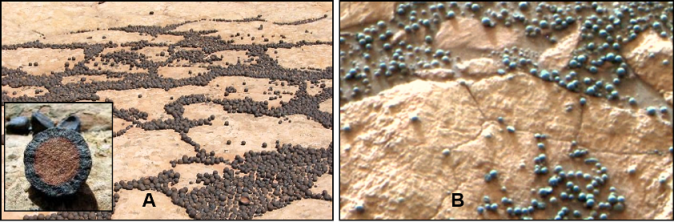
Another discovery is what are called Martian blueberries on the surface of some of Martian sedimentary rocks (Figure 4). These BB-sized spherical blueberries are not really blue but rather are small concretions of iron oxide and are analogous to Moqui marbles found on Earth. They are found on the surface and within layers of sedimentary rocks.
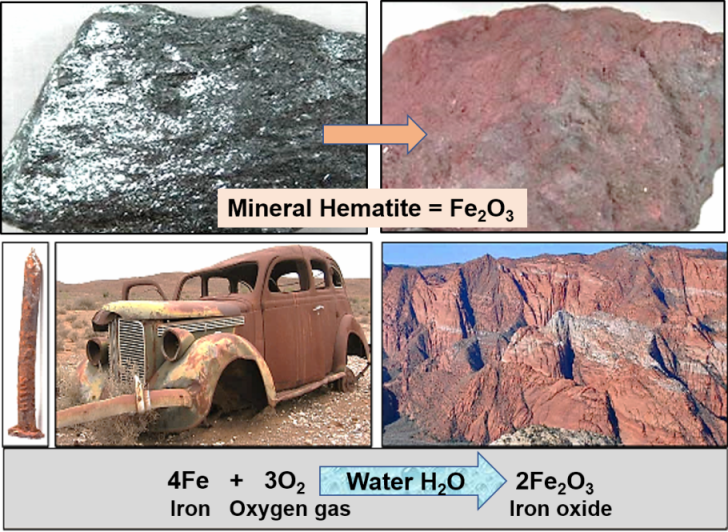
Oxidation on Earth
Oxidized iron in the form of red beds, such as the Navajo Sandstone, commonly occur on Earth. The chemical reaction of oxidation, which requires water and oxygen gas, is commonly recognized as rust (Figure 5). However, up until between 2 and 2.5 billion years ago, the rock record indicates that oxidation was not a common reaction on Earth. There are virtually no red beds known from the rock record previous to that time, and this indicates that our ancient atmosphere lacked significant permanent amounts of oxygen gas.
Life on Mars
A significant question, therefore, is what is the main source for oxygen gas on Earth and Mars? The current atmosphere on Earth contains approximately 21 percent oxygen gas. In stark contrast, the current atmosphere of Mars contains approximately 0.17 percent oxygen gas. Overall, the atmospheric surface pressure on Earth is 1,013 millibars whereas on Mars it is a very low 7 millibars.
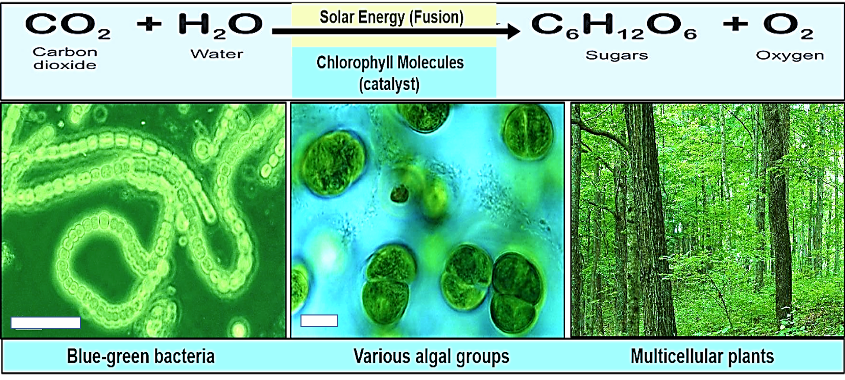
Virtually all oxygen gas on Earth is produced as a byproduct of photosynthesis by blue-green bacteria, various groups of algae, and green plants (Figure 6). Other sources of oxygen gas include electrolysis of water, decomposition of hydrogen peroxide, and catalytic conversion of ozone in the upper atmosphere. However, these processes provide a negligible amount of oxygen compared with that produced by photosynthesis.
At the present time, a source for oxygen gas on Mars has not been determined. One speculative possibility is that it was produced in the same way as on Earth: by the process of photosynthesis. As described previously in Part 3, preserved geologic features on Mars indicate presence of a hydrosphere and atmosphere early in the planet’s history. These conditions are similar to what has been recognized on Earth (Figure 7) and therefore suggest that life could also have been present on Mars.
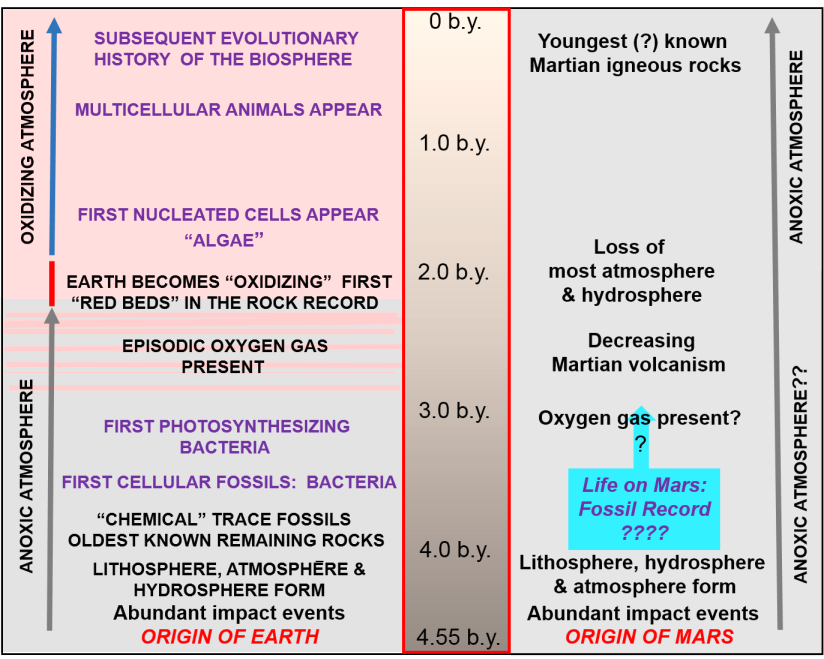
Currently, the oldest fossil record of life on Earth dates back about 4 billion years ago and consists of unicellular bacteria and bacteria-like fossils (see Part 2). However, the oldest record of organisms capable of photosynthesis, and therefore producing oxygen gas, are known as cyanobacteria (blue-green bacteria). Currently, we know that they appear in the fossil record between 3.2 and 3.5 billion years ago (Figure 8).
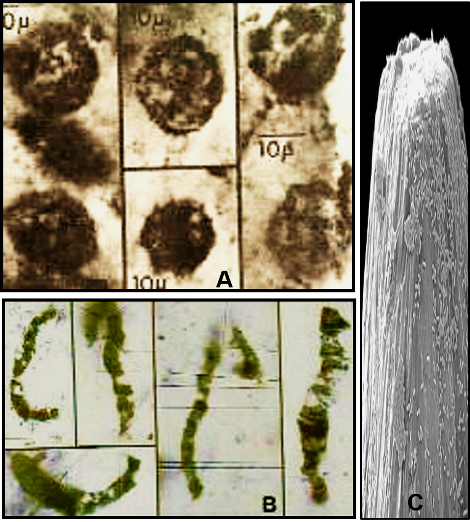
From a historical perspective, it is notable that such ancient microfossils were not discovered on Earth until the mid-1950s, and definitive results were not published until the mid-1960s (Barghoorn, E. S. and Tyler, S. A., 1965: Microorganisms from the Gunflint Chert. Science, vol. 147, p. 563–577). This initial and unexpected discovery from 2-billion-year-old rocks in the Great Lakes region sparked a paleontological equivalent of a gold rush with new discoveries of fossils reported from ancient rocks on many continents, which have extended the known record of life back to about 4 billion years ago.
Finding preserved microscopic fossils on Mars would most likely involve searching for siliceous rocks deposited in water such as those in the Gale Crater. If any such siliceous rocks can be found on Mars, they will have to be thin-sectioned and studied under relatively high magnification in order to search for fossilized microbes (Figure 9). So it is likely that these rocks will have to be brought back to Earth to be studied.
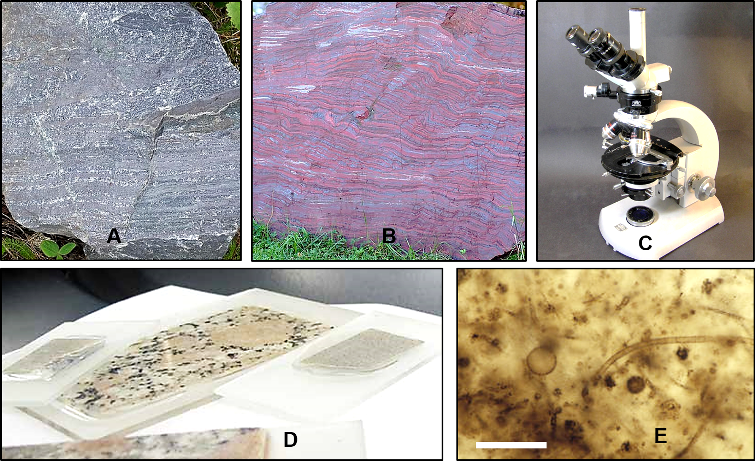
Based on what is known about the history of Mars, such rocks would have had to form earlier than 3 billion years ago. That is the time interval when Mars had a protective magnetic field, an atmosphere, and a hydrosphere. Thus, active geological processes such as the rock and hydrologic cycles were operating on its surface, and they produced the geologic features illustrated in Part 3.
After that time, likely due to reduction in radioactive isotopic decay, there was a significant decrease in interior heat production and cessation of most volcanic activity. Because Mars has a lower gravitational pull and volcanic outgassing had essentially stopped, the result was a loss of its atmosphere and hydrosphere into space. In effect, Mars died internally and began its journey to becoming a very cold and dry planet (Figure 10).
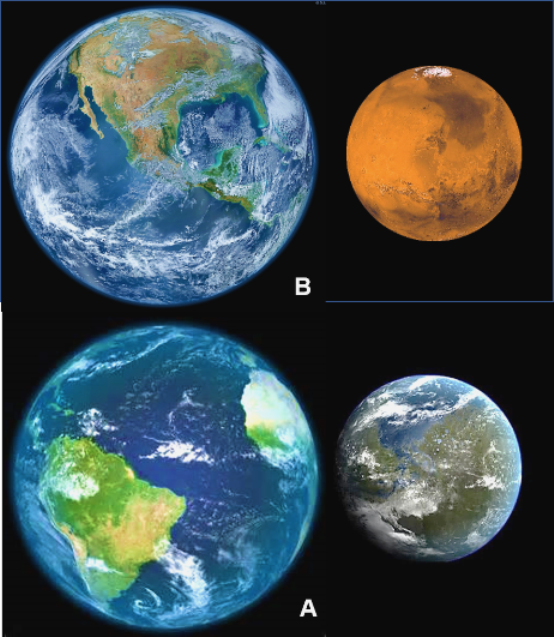
Table 1 provides a summary and interpretation of the evidence discussed in all four articles. A key consideration is the fact that geological evidence indicates that for the first billion years of their respective histories Earth and Mars seem to have been very similar. That is the time interval when life formed on Earth, and perhaps it did so on Mars as well.

Astronomers have estimated that the universe and the Milky Way Galaxy formed over 13.5 billion years ago. Within the Milky Way, our Solar System formed “only” about 4.5 billion years ago, so it is relatively young compared to the oldest stars and other objects within the universe and Milky Way.
It has also been estimated that there are over 100 billion planets within the Milky Way. Even if just a fraction of those planets exist within the habitable zone of their respective solar systems, there are presumably a very large number of planets that could support carbon-based life like here on Earth.
Therefore, given that some of these habitable planets are significantly older than Earth, are there any advanced life forms or civilizations on some or many of these planets? That leads to more speculation as to why is there no evidence of or communication from such civilizations.
Here are some possibilities: Constraints such as time and distance prevent such communication, any such civilizations are purposely avoiding any type of contact, advanced civilizations are attempting to contact us but our technology is too primitive to recognize any such attempt, and the most depressing possibility is that such civilizations developed technologically as humans have been doing on Earth but ended up destroying themselves in the process. Perhaps we humans are rushing headlong down that same destructive pathway.
Acknowledgments
Geology is the study of Earth’s physical and biological history. Such study often indicates that ancient events and features have significance for determining future events and features. Conversely, present events and features can be used to interpret and understand past events and features. So it is with this series of four articles in the Independent.
For me, the seed event for these articles was planted by two of my UCLA Professors, Dr. Preston Cloud and Dr. William Schopf. During the 1960s and 1970s, when I was a student there, they were both deeply involved with the rapidly growing searches and subsequent discoveries of ancient fossils from Precambrian rocks. At that time this search had become a major area of research. They both shared their discoveries with lectures and publications.
Jumping ahead to the mid-1990s, NASA began serious explorations of Mars. By that time, I was a geology professor at San Diego State University and attended an invited lecture by one of my colleagues, professor Gary Peterson. He noted that the red color of Mars was due to oxidation of iron minerals. His point was that the oxygen needed for oxidation may have been produced by photosynthesis, and if so, that would indicate that life existed on Mars at some time in the past. I consider that lecture as germinating the seed planted back at UCLA.
Beginning with those 1990s discoveries and continuing to the present time, NASA and other space agencies have provided a wealth of information about physical conditions that existed early in Martian history. I consider this information to represent the water and nutrients that have allowed me to write these articles. Hopefully, I have not created a weed from my speculations about previous and current evidence.
Finally, I thank Smilla Bithell and Yvonne Lynott for very helpful editorial suggestions that have improved this article.
Articles related to “Life on Earth and Mars: Was there life on the red planet?”
Life on Earth and Mars: Where have we been and where are we going



